
In the event that calcium and phosphate are needed for other functions, bone tissue can be broken down to supply the blood and other tissues with these minerals. More than 90 percent of the calcium and phosphate that enters the body is incorporated into bones and teeth, with bone serving as a mineral reserve for these ions.
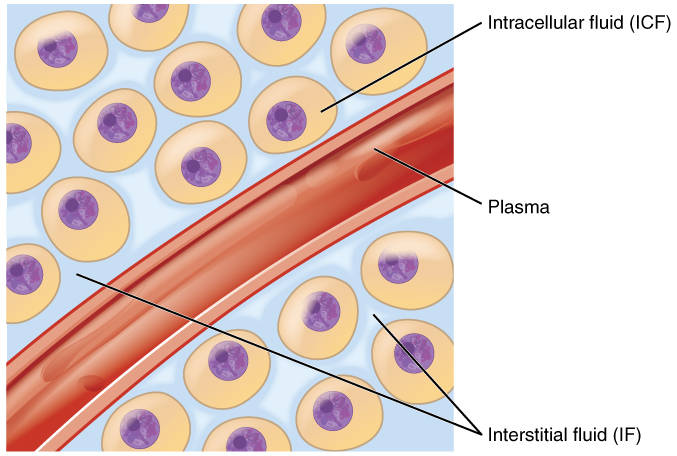
These ions enter the body through the digestive tract. These six ions aid in nerve excitability, endocrine secretion, membrane permeability, buffering body fluids, and controlling the movement of fluids between compartments. In terms of body functioning, six electrolytes are most important: sodium, potassium, chloride, bicarbonate, calcium, and phosphate. All of the ions in plasma contribute to the osmotic balance that controls the movement of water between cells and their environment.Įlectrolytes in living systems include sodium, potassium, chloride, bicarbonate, calcium, phosphate, magnesium, copper, zinc, iron, manganese, molybdenum, copper, and chromium. Still others aid in releasing hormones from endocrine glands. Other ions help to stabilize protein structures in enzymes. Some ions assist in the transmission of electrical impulses along cell membranes in neurons and muscles. The body contains a large variety of ions, or electrolytes, which perform a variety of functions. Describe the role of aldosterone on the level of water in the body.Identify the predominant extracellular anion.Name the disorders associated with abnormally high and low levels of the six electrolytes.List the role of the six most important electrolytes in the body.By the end of this section, you will be able to:


 0 kommentar(er)
0 kommentar(er)
